insights
Prebiotic Foods for Postbiotic Abundance

Read Time: 6 Minutes
A balanced and healthy gut microbiome is essential for optimal immune function and resiliency, and both the intestinal microbial community as well as their metabolites impact host health system wide. Supporting commensal gut microbes through a diverse, plant-based diet of fibers and prebiotic foods may ensure abundance of not only beneficial bacteria but also those fermentation by-products, or postbiotics, that demonstrate nutritional, metabolic, and immune health benefits.1
Evidence supports the consumption of plant foods for positive effects on health, including lowering blood pressure, cholesterol levels, body mass, and inflammation.1,2 Whole food, plant-based diets are also effective therapeutic strategies for combatting chronic diseases and have been suggested to:
- Decrease risk of cardiovascular disease, diabetes, obesity, and cancer.2-4
- Slow the progression of chronic kidney disease.2
- Improve depression and anxiety symptoms as an adjunctive therapy.5
- Provide benefits for those with chronic fatigue, pain, and insomnia.5
- Aid in the prevention of ulcerative colitis relapse.6
The list goes on, but what are the mechanisms behind the benefits of a plant-based diet, or adding more plant foods to a nutrition plan? The relationship between fiber-rich foods that include prebiotics and the gut microbiota’s fermentation by-products is an important component.
Soluble and insoluble fibers are indigestible carbohydrates that are available in plants and plant-based foods. While insoluble fibers benefit a healthy system by aiding in the efficient elimination of wastes, soluble fibers promote fermentation by the gut microbial community. And prebiotics, nutrients selectively used by host microorganisms resulting in health benefits,7,8 are present in plant-based, fiber-rich foods. In order for substrates to be classified as a prebiotic, certain criteria are required, such as:7
- Documented beneficial health effects.
- Selective microbiota-mediated mechanisms.
- May include non-carbohydrate substances.
Some examples of prebiotics that naturally exist in foods are:9
- Fructans, including inulin and fructooligosaccharide.
- Galactooligosaccharides and trans-galactooligosaccharides.
- Resistant starches and oligosaccharides.
- Polyphenols and cocoa-derived flavanols.
And while a diverse, whole food, plant-based diet that is fiber-rich contains an array of prebiotics, some prebiotic foods that are selective to known beneficial gut bacteria include asparagus, bananas, barley, beans, sugar beets, chicory, garlic, honey, human and cow’s milk, onions, peas, rye, seaweed and microalgae, soybeans, tomatoes, and whole grain wheat.9
Prebiotics “feed” the commensal gut microbial community. The beneficial bacteria ferment these non-digestible compounds and obtain energy through the degradation process to flourish in their activity and growth. In this way, prebiotics can influence the landscape of the gut microbiome and benefit overall health through maintaining or increasing the population of health-protective gut microbes.9
Adding one or more prebiotic foods to a diet plan can have significant health benefits. In a 2015 randomized controlled trial, participants replaced refined wheat with whole grain wheat in their diet and noted a significant increase in ferulic acid, an antioxidant and anti-inflammatory agent, as well as an increase in its metabolite dihydroferulic acid.10 In 2019, a small, single-group design trial evaluated health impacts in healthy individuals after the daily consumption of vegetables rich in inulin-type fructans (ITF).11 Assessments included nutrient intake, fecal microbiota composition, microbial fermentation, gastrointestinal symptoms, and food-related behavior. During the two-week intervention period, participants followed a controlled diet that was based on ITF-rich vegetables and included an intake of 15 grams of ITF per day. At the end of treatment, one primary microbial modification included an increased proportion of the health-promoting Bifidobacterium genus.11,12 In addition, participants demonstrated the following:11
- Greater satiety.
- A reduced desire to eat sweet, salty, and fatty foods.
- An increased positive attitude toward some inulin-rich vegetables.
- Improved intestinal discomfort by the end of the intervention.
Health Benefits of Postbiotics
As commensal gut microbes break down prebiotics through fermentation, various metabolites and by-products are generated that contribute to system-wide health benefits. These postbiotics demonstrate positive health effects from their potential immunomodulatory, anti-inflammatory, antioxidant, and anti-cancer properties13,14 and possibly help to inhibit pathogens.15 The concept of postbiotics is based on the observation that a host’s health benefit from gut microbes is due in part to their secretion of metabolites and fermentation by-products.13,16 As this science continues to evolve, the International Scientific Association for Probiotics and Prebiotics (ISAPP) panel recently offered a standardized definition of a postbiotic as a preparation of inanimate microorganisms and/or their components that demonstrate host health benefit.8
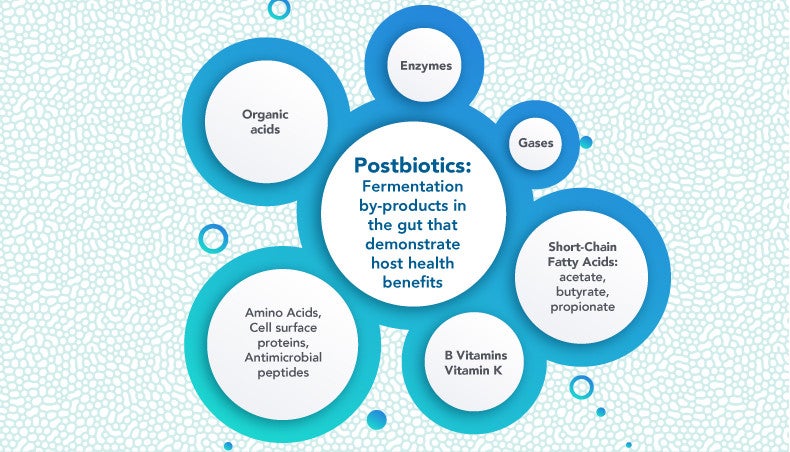
The short-chain fatty acids (SCFAs) butyrate, propionate, and acetate are examples of postbiotics. They are products of prebiotic degradation and have a range of health benefits, from providing an energy source to human colonocytes, regulating potential anti-cancer activity, enhancement of the intestinal barrier, and satiety signaling to positively impacting glucose and energy homeostasis and promoting the growth of other bacteria through bacterial cross-feeding.19-21
SCFA abundance may also be involved in the prevention of chronic conditions. A 2019 cohort study evaluated the fecal samples of 301 one-year-olds and suggested significant associations between the levels of SCFAs due to an infant’s diet and development of atopy, allergy, and asthma.22 The analysis indicated that those children with the highest levels of butyrate and propionate in their feces at the age of one year had significantly less atopic sensitization and were less likely to have asthma between three and six years of age.22 In addition, those with the highest levels of butyrate were also less likely to have a reported diagnosis of food allergy or allergic rhinitis.22
Individual Gut Microbiota Composition
An important consideration regarding the metabolism of prebiotics, their resulting postbiotics, and subsequent health benefits is the variation of gut microbial composition between populations and individuals. For polyphenols, for example, certain fermentation products will only be present, and their health benefits fully realized, if specific bacteria or groups of bacteria are present within a person’s gut landscape.9,15,19,23 One of the most well-studied interindividual variations is the metabolism of the polyphenol daidzein.19 Depending on the gut microbiota composition, this soy isoflavone may be metabolized via different pathways, resulting in different fermentation products, including the presence or absence of equol.19 Equol is a beneficial metabolite that promotes estrogenic and antioxidant activities.24
Clinical Considerations
Personalized nutrition interventions are a cornerstone of functional medicine care. Combatting chronic diseases through modifiable lifestyle factors such as diet may optimize gut function and improve overall health. Due to the individuality of a patient’s gut microbial landscape, a diverse diet of whole plant foods and other fiber-rich sources, including fermented foods, helps to ensure a varied intake of prebiotic fibers and may maximize the abundance of beneficial metabolites. Even introducing one or more prebiotic foods to a patient’s current nutrition plan can help feed those beneficial gut microbes and contribute to system-wide health benefits.
Learn more about supporting a healthy gastrointestinal (GI) tract and gut microbiome at IFM’s upcoming GI Advanced Practice Module (APM).
Related Articles & Podcasts
Health, Nutrition, and the Role of the Microbiome
Emerging Concept: Optimizing the Pediatric Microbiome
References
- Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013;17(2):61-66. doi:10.7812/TPP/12-085
- Adair KE, Bowden RG. Ameliorating chronic kidney disease using a whole food plant-based diet. Nutrients. 2020;12(4):1007. doi:10.3390/nu12041007
- Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640-3649. doi:10.1080/10408398.2016.1138447
- Sterling SR, Bowen SA. The potential for plant-based diets to promote health among Blacks living in the United States. Nutrients. 2019;11(12):2915. doi:10.3390/nu11122915
- Null G, Pennesi L. Diet and lifestyle intervention on chronic moderate to severe depression and anxiety and other chronic conditions. Complement Ther Clin Pract. 2017;29:189-193. doi:10.1016/j.ctcp.2017.09.007
- Chiba M, Nakane K, Tsuji T, et al. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: a single-group trial. Perm J. 2019;23:18-220. doi:10.7812/TPP/18-220
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491-502. doi:10.1038/nrgastro.2017.75
- Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics [published correction appears in Nat Rev Gastroenterol Hepatol. 2021;18(9):671] [published correction appears in Nat Rev Gastroenterol Hepatol. 2022;19(8):551]. Nat Rev Gastroenterol Hepatol. 2021;18(9):649-667. doi:10.1038/s41575-021-00440-6
- Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi:10.3390/foods8030092
- Vitaglione P, Mennella I, Ferracane R, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. 2015;101(2):251-261. doi:10.3945/ajcn.114.088120
- Hiel S, Bindels LB, Pachikian BD, et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr. 2019;109(6):1683-1695. doi:10.1093/ajcn/nqz001
- O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. 2016;7:925. doi:10.3389/fmicb.2016.00925
- Zólkiewicz J, Marzec A, Ruszczynski M, Feleszko W. Postbiotics—a step beyond pre- and probiotics. Nutrients. 2020;12(8):E2189. doi:10.3390/nu12082189
- Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, et al. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105-114. doi:10.1016/j.tifs.2018.03.009
- Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C. Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci. 2019;20(19):4673. doi:10.3390/ijms20194673
- Collado MC, Vinderola G, Salminen S. Postbiotics: facts and open questions. A position paper on the need for a consensus definition. Benef Microbes. 2019;10(7):711-719. doi:10.3920/BM2019.0015
- Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. doi:10.1186/s40168-019-0704-8
- Toca MDC, Burgos F, Fernández A, et al. Gut ecosystem during infancy: the role of “biotics.” Arch Argent Pediatr. 2020;118(4):278-285. doi:10.5546/aap.2020.eng.278
- Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1-24. doi:10.1007/s00394-017-1445-8
- Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr. 2019;59(Suppl 1):S130-S152. doi:10.1080/10408398.2018.1542587
- Venter C, Eyerich S, Sarin T, Klatt KC. Nutrition and the immune system: a complicated tango. Nutrients. 2020;12(3):E818. doi:10.3390/nu12030818
- Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy.2019;74(4):799-809. doi:10.1111/all.13660
- Cortés-Martín A, Selma MV, Tomás-Barberán FA, González-Sarrías A, Espín JC. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol Nutr Food Res. 2020;64(9):e1900952. doi:10.1002/mnfr.201900952
- Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11(9):2231. doi:10.3390/nu11092231




